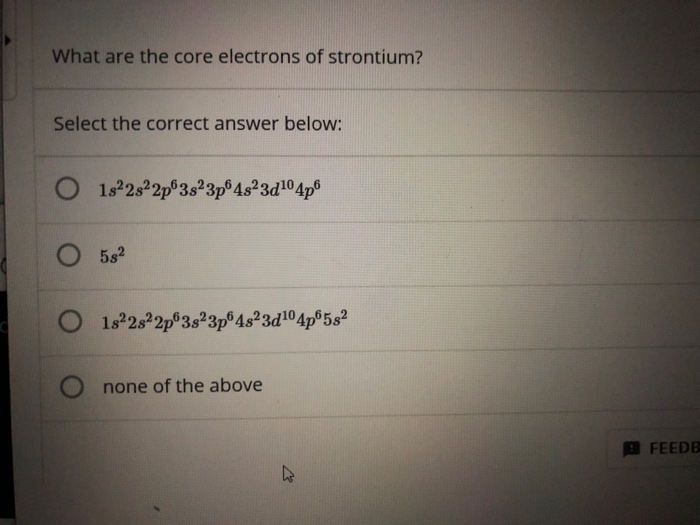
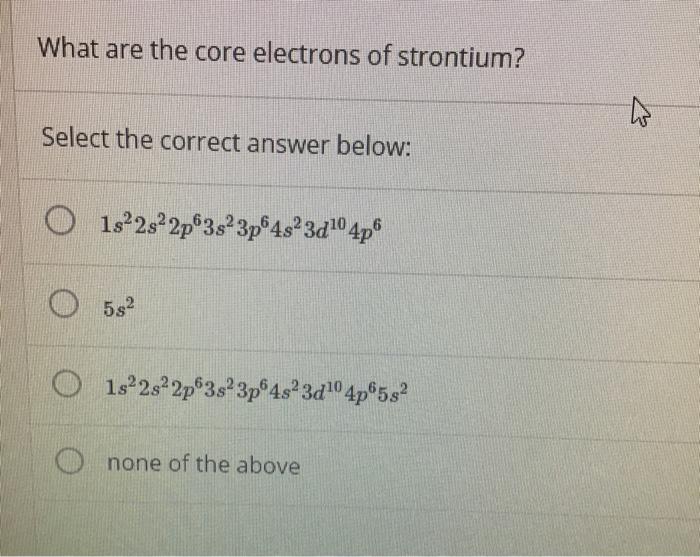
What Are the Core Electrons of Strontium
Number of Neutrons most commonstable nuclide. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
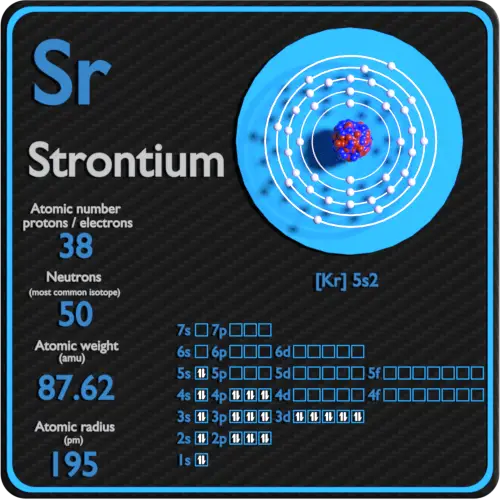
Strontium Protons Neutrons Electrons Electron Configuration
Krypton has the electron configuration 1s22s22p63s23p64s23d104p6.

. As we have already come to know the electronic configuration of strontium therefore it is written as Kr 5s 2. Sr 2e Sr 2. Therefore the number of electrons in neutral atom of Strontium is 38.
That is the number of electrons in strontium is thirty-eight. It has an atomic number of 38 an atomic mass of 88 one oxidation state 2 and four naturally occurring isotopes 84 Sr 86 Sr 87 Sr 88 Sr of which 88Sr is the most abundant at 826 of the total mass. There must be equal numbers of these particles because here the atom was electronically neutral.
Strontiums full electron configuration is 1s22s22p63s23p64s23d104p65s2. Strontium-90 is especially deadly since it has a relatively long half-life is strongly radioactive and is absorbed by the body where it accumulates in the skeletal. In order to write the Sr electron configuration we first need to know the number of electrons for the Sr atom there are 38 electrons.
The higher the associated electronegativity number the more an element or compound attracts electrons towards it. So it has 38 protons and 38 electrons if its neutral. Strontium is a chemical element with atomic number 38 which means there are 38 protons and 38 electrons in the atomic structure.
Strontium in the environment exists in four stable isotopes84Sr read as strontium eighty-four86Sr87Sr88Sr. Chemistry questions and answers. Below is the electronic diagram of the Strontium atom Distribution of electrons over energy levels in the Sr atom.
Possible oxidation states are 2. Strontium is the chemical element with the symbol Sr and atomic number 38. Select the correct answer below.
Strontium is a group 2 element and so has 2 valence electrons. What are the core electrons of strontium. The nucleus is composed of protons and neutrons.
Valence electrons present in the last shell of strondium. Naturally occurring strontium is not radioactive and is either referred to as stable strontium or strontium. Comprehensive information for the element Strontium - Sr is provided by this page including scores of properties element names in many languages most known nuclides and technical terms are linked to their definitions.
In general an atoms electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The chemical symbol for Strontium is Sr. 1s 2s22p63sº 3p 4s 3210 4p O 582 O 1s 2s 2p63s 3p64s3d1º 4p 5s2 O none of the above.
Strontium compounds are used in making ceramics and glass products. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. When we write the c.
We tried to answer How Many Electrons are in Strontium. Therefore the configuration for the valence electrons in strontium is 5s2. O 1922s22p3523p453210 4p 0 58² O 1522s22p 3s 3p4823d104p582 O none of the above FEEDE.
Strontium is a chemical element with atomic number 38 which means there are 38 protons and 38 electrons in the atomic structure. The chemical symbol for Strontium is Sr. Reduced electronic configuration Sr.
Full electron configuration of strontium. The electron configuration of strontium ion Sr 2 is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. The metal forms a dark oxide layer when it is exposed to air.
Select the correct answer below. As we have mentioned before to know the valence electrons one can check how many electrons are there in the outer shell. Therefore a strontium atom will have two electrons in the first shell eight in the 2nd orbit eighteen electrons in the 3rd shell eight electrons in the 4th shell and the remaining two electrons will be in the 5th shell.
The atomic number of strontium is 38. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table calcium and. An alkaline earth metal strontium is a soft silver-white yellowish metallic element that is highly chemically reactive.
Electron Configuration and Oxidation States of Strontium. These core electrons are stable and do not take part in bonding while the remaining electrons are valence electrons. The element was eventually isolated by Sir Humphry Davy in 1808.
Strontium is a naturally occurring element found in rocks soil dust coal and oil. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom.
Strontium-90 a radioactive isotope of strontium is a common product of nuclear explosions. It has a half-life of about 288 years and decays into yttrium-90 through beta decay. And thus in a strontium nucleus there are 38 massive positively charged particles 28 protons this is what defines the atom as strontium and around the nucleus there are NECESSARILY 28 negatively charged particles that we call electrons are conceived to whizz about.
Strontium was recognized as a new element in 1790 when Adair Crawford and his colleague William Cruickshank analyzed a mineral sample from a lead mine near Strontian Scotland. Electronic configuration of the Strontium atom in ascending order of the levels. The electronegativity of Strontium is.
What are the core electrons of strontium. Thus the rest of the 36 electrons are core electrons. Electron configuration of Strontium is Kr 5s2.
Chemistry questions and answers. Last shell of strondium is 5s2 so strondium atom has 2 valence electrons. So it has 2 electrons in the outer shell so valence electrons Strontium Electron Configuration have is 2.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2. The isolation was done by the electrolysis of a mixture containing strontium chloride and mercuric oxide. Strontium has a electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2.
Number of Electrons with no charge. This electron configuration shows that strontium ion have four shells and the last shell has eight electrons 4s 2 4p 6 and the strontium ion Sr 2 has acquired the electron configuration of krypton. Strontium is most similar chemically to the heavier alkali earth elements Calcium and Barium.
Solved What Are The Core Electrons Of Strontium Select The Chegg Com

How Many Valence Electrons Does Strontium Sr Have
Solved What Are The Core Electrons Of Strontium Select The Chegg Com

Rb Electron Configuration Rubidium Ion Electron Configuration Electrons Configuration
Comments
Post a Comment